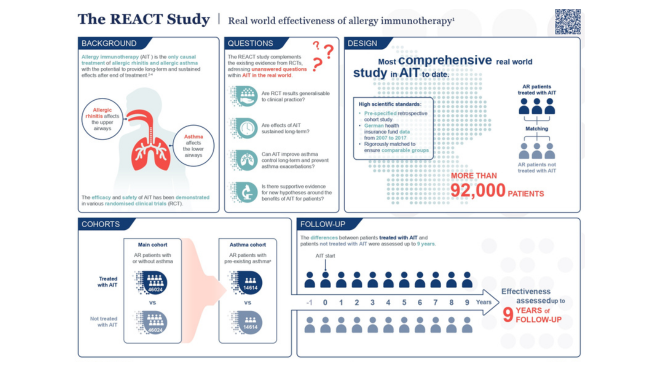
From clinical to real-world evidence
Allergy immunotherapy (AIT) has evolved significantly over the last decade, triggered by the need for more evidence-based medicine. ALK has worked with the allergy community to develop the fields' most robust clinical trial programme, enrolling over 25,000 patients, enabling prescribing decisions to move from experience-based to evidence-based. But ideal-world evidence alone can only tell you so much.
That is where our programme Real-world Evidence in Allergy (REWEAL) comes in, demonstrating how AIT works in the real world.